Abstract
Background
Waldenström's macroglobulinemia (WM) is a rare low grade B-cell NHL, with generally excellent outcomes underpinned by treatment with Rituximab combination therapies and covalent BTK inhibitors (cBTKi). However, these options are not curative and there are very little data to guide treatment options or a clear understanding of prognosis of relapsed/refractory (R/R) WM post cBTKi. PD-1 inhibition has been shown to be highly effective in classical Hodgkin lymphoma, and soluble PD-1 ligands have been implicated in T-cell regulatory function in the WM microenvironment. There is therefore a clear rationale to investigate the efficacy of PD-1 blockade in WM.
Aim
To determine the safety, tolerability and efficacy of pembrolizumab in combination with rituximab for the treatment of relapsed/refractory WM following prior cBTKi exposure.
Methods
PembroWM is a phase II, non-randomised, single arm, open label NCRI UK-wide study (NCT03630042) enrolling patients (pts) with R/R WM who had received 1 or more prior lines of treatment, who were not refractory to rituximab based therapy (progressing on or relapsing within 6 months of a rituximab containing regimen). Eligible pts accross 6 sites received rituximab 375mg/m2 weekly for 4 weeks, then 3 monthly (total of 8 doses) and pembrolizumab 200mg IV on a 3-weekly cycle, for a maximum of 18 cycles. The primary end point was the percentage of pts achieving an overall response rate (ORR) of greater than 25% reduction in serum IgM at 24 weeks post treatment. Secondary end points included safety and tolerability, response rates (CR/VGPR) , progression free survival (PFS) and overall survival (OS) at 1 year. Disease response was assessed with serum paraprotein/immunofixation, BM and CT imaging at 24 weeks and 1 year using the International Working Group for WM response criteria. The trial required recruitment of 42 patients with a 55% response rate to meet the primary end point. The trial closed early due to low recruitment rate with a total of 17 patients with a revised response requirement of 9 patients to meet primary end point.
Results
17 patients were registered with median age of 70 years (range 43-84), 83% were male, 94% had a PS of 0-1, and the median number of lines of previous treatment was 3 (range 1-6). The majority of patients had a cBTKi as their last treatment prior to enrollment (only 2 patients were BTK naïve). 8 patients were positive for the MYD88 L265P mutation, and 4 patients had mutations in BTKC481. Median IgM levels were 40 g/L (range 2.5-72g/L) with 53% of patients exhibiting WM cells in bone marrow and extra nodal disease in 6%.
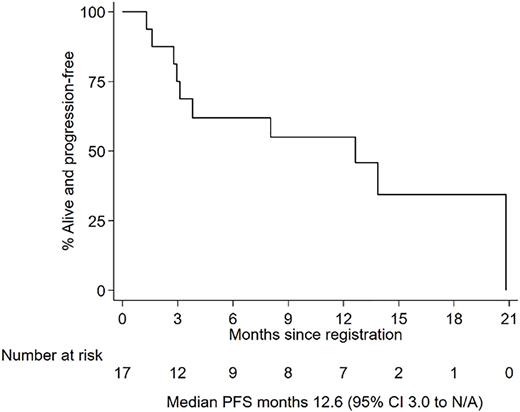
Median follow up is 15.1 months (0.5 - 24.9). ORR at 24 weeks post treatment was 47.1% (60% CI 34.5-60%) with 6% attaining VGPR, 18% PR, 18% MR and in 6% response was unavailable but there was evidence of MR at week 12. 47% were non-responders; 35% progressed, 12% had stable disease and one patient withdrew during cycle 1 so was non-evaluable. ORR at 52 weeks post treatment was 36.4% (95% CI 10.9-69.2%) with 9% obtaining a VGPR, 27% PR and nil achieving CR. Median PFS was 12.6 months (95% CI 3-n/a) and median OS was not reached.
5 patients completed 18 cycles of treatment. 6 progressed, 3 withdrew due to AE, 2 withdrew consent and one withdrew to an unrelated clinician decision. The AE leading to withdrawal were immune thrombocytopenia (one), and infection in the other two (including one COVID-19 infection)
The majority of adverse events were grade 1-2 with the commonest events involving anaemia (29%), fever (29%), infusion related reactions (35%) and raised creatinine (29%)., There were only 2 grade 3 infections. 1 patient experienced an embolic stroke and 1 patient died from respiratory failure; both events were considered unrelated to treatment.
Conclusions PEMBROWM is the first study to investigate checkpoint inhibition in WM, and demonstrates the safety and tolerability of the combination of pembrolizumab and rituximab, with encouraging efficacy in a heavily pre-treated population. The recruitment challenges highlighted the combination of reduction in research activity during the COVID-19 pandemic, the difficulties of recruiting to an investigator-led study in a rare indication and the timing of availability/effectiveness of cBTKi in WM in the United Kingdom. A randomised study would be required to evaluate the additional benefit that PD-1 blockade is adding to rituximab monotherapy in a highly relapsed/refractory patient population.
Disclosures
Kothari:MSD: Research Funding; Beigene: Consultancy, Speakers Bureau. Eyre:Medscape: Speakers Bureau; PeerView: Speakers Bureau; Loxo Oncology @ Lilly: Membership on an entity's Board of Directors or advisory committees, Other, Speakers Bureau; Secura Bio: Membership on an entity's Board of Directors or advisory committees; Kite Pharma: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Gilead: Honoraria, Membership on an entity's Board of Directors or advisory committees; Abbvie: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Incyte: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; AstraZeneca: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Roche: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Beigene: Membership on an entity's Board of Directors or advisory committees, Research Funding. Rismani:Astra Zeneca: Honoraria, Speakers Bureau. McCarthy:Janssen, astra zeneca, amgen: Honoraria. Lewis:Janssen: Consultancy, Other: Speakers fees; travel grant; Eli Lilly and Company: Consultancy; Roche: Consultancy; Kite: Consultancy, Other: Travel grant; Beigene: Consultancy. Clifton-Hadley:Astra Zeneca, GSK, Pfizer, MSD, BMS, Amgen, Millennium Takeda: Other: CRUK and UCL CTC have received research funding in the past 24 months, Research Funding. Pratt:Takeda: Consultancy; Janssen: Consultancy; Gilead: Consultancy; BMS/Celgene: Consultancy; Binding Site: Consultancy; Amgen: Consultancy. Owen:Beigene: Honoraria, Membership on an entity's Board of Directors or advisory committees; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Astra-Zeneca: Honoraria. D'Sa:Janssen; Sanofi; BeiGene: Consultancy; janssen; beigene: Other: grant funding .
OffLabel Disclosure:
Pembrolizumab - not on label for the treatment of waldenstrom macroglobulinaemia
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal